What Does the Vsepr Theory Tell About a Molecule Apex
The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. The premise of VSEPR is that the valence electron pairs surrounding an atom.

6 3 Molecular Shape Introductory Chemistry
Advertisement Advertisement New questions in Chemistry.
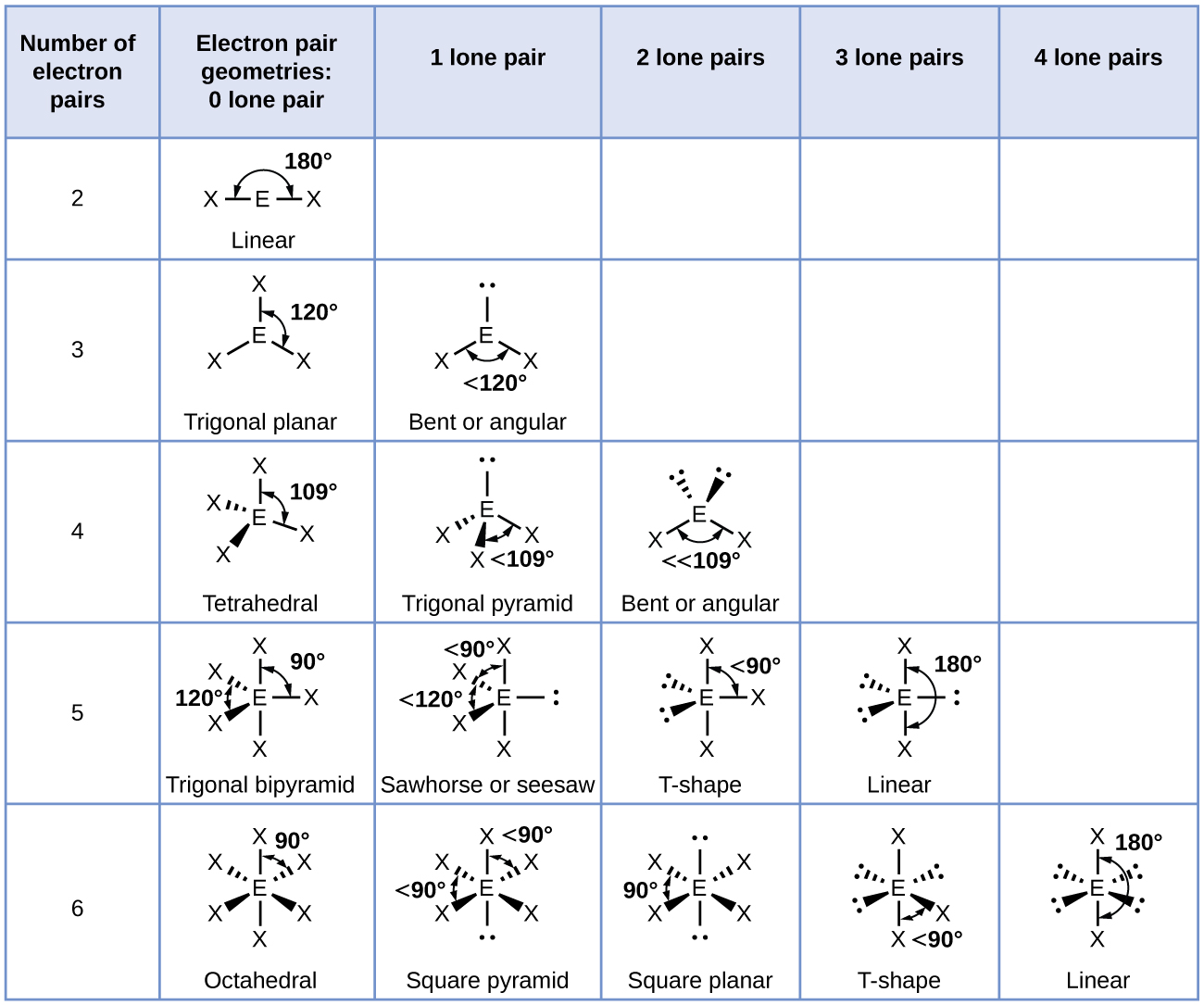
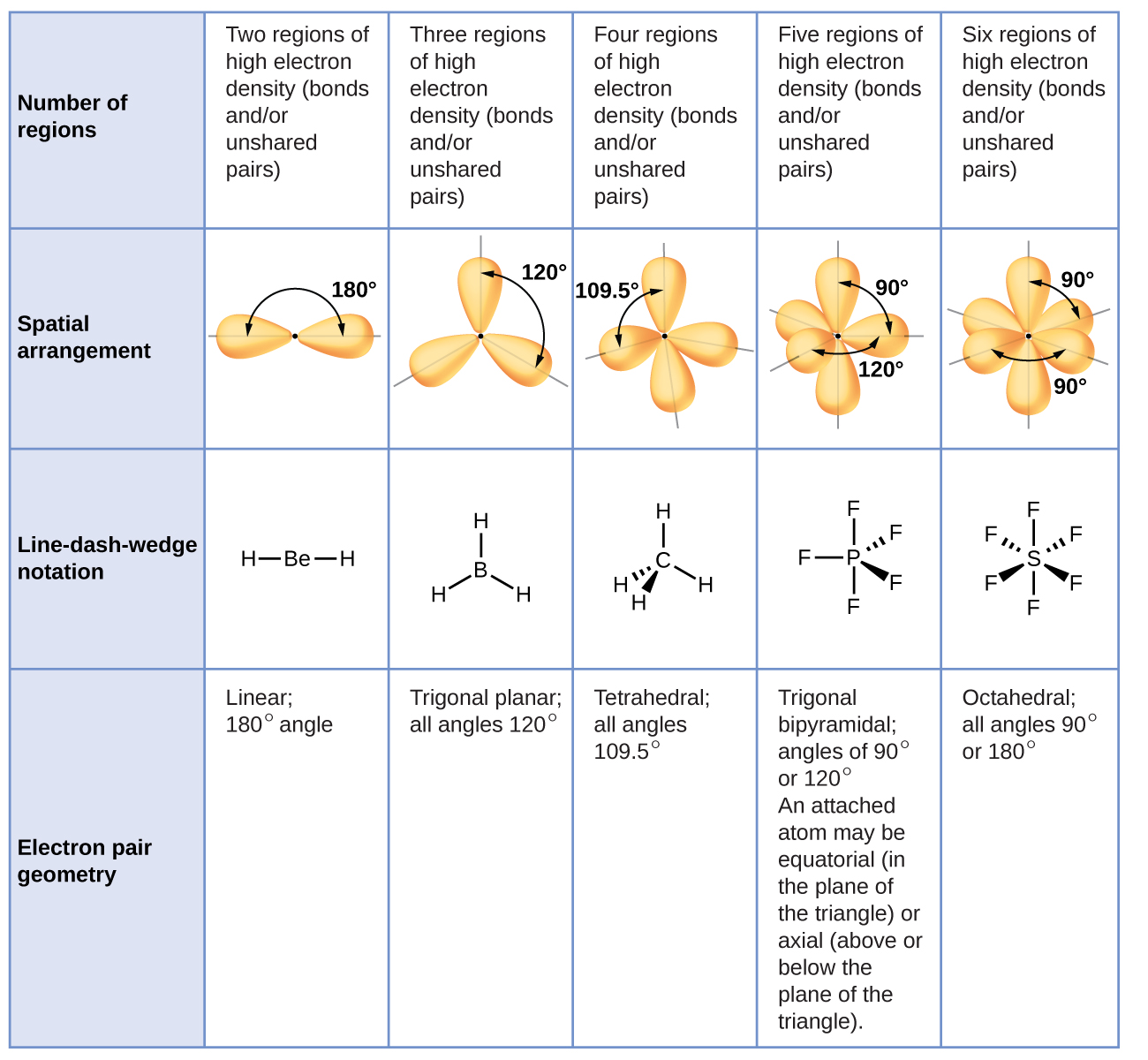
. Predicting the Shapes of Molecules. VSEPR - Valence shell electron pair repulsion theory predicts the shape of a molecule. In VSEPR theory the terminal atom locations are structurally equivalent in each of the linear trigonal planar and tetrahedral electron-pair.
What is the correct name for Na₂O. The VSEPR theory tells us that molecules take on regular and unique shapes because valence electrons push each other away. What does VSEPR theory tell about a molecule.
Learn vocabulary terms and more with flashcards games and other study tools. VSEPR counts the number of electron pairs around a central atom both bonding and non-bonding and assigns a shape according to the Platonic solids. Youre totally right that the triple bond causes more repulsive forces but since there are only two groups the furthest the triple bond can push away the single bond is into a line.
VSEPR Theory correctly predicts it as linear because there are only two electron groups around the carbon atom. A P E X. 5 What defines the shape of a molecule.
Steps for using VSEPR Theory-draw a lewis dot diagram-predict the geometry around the central atom-name the molecular shape. It is a fact that electron pairs arranged around a central atom repel each other hence vesper. You can also predict _____ _____ and _____ _____ by using VSEPR Theory.
Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure including approximate bond angles around a central atom of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. What does the VSEPR theory tell about a molecule. 10 How does the shape of a molecule affect its polarity.
What is the VSEPR theory. The bond angle becomes smaller as the number of lone pairs increases. Start studying VSEPR Theory.
This arrangement of the atom shapes the geometry of the resulting molecule. Using this theory you can determine what shape a molecule will take in. The theory was first presented by Sidgwick and Powell in 1940.
Compounds are classified by steric number see sets below and by the number of lone pairs on the central atom. The geometry it will have. See this older answer.
Valence shell electron pair repulsion theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. The VSEPR theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized. Electrons exist in _____ also known as.
VSEPR models are based on the concept that electrons around a central atom will configure themselves to minimize repulsion and that dictates the geometry of the molecule. The VSEPR Theory Valence Shell Electron Pair Repulsion Theory is based on the fact that there is a repulsion between the pairs of valence electrons in all the atoms and the atoms will always try to arrange themselves in a manner in which the electron pair repulsion is minimized. It is also named the Gillespie-Nyholm theory after its two main developers Ronald Gillespie and Ronald Nyholm.
6 How does the VSEPR theory predict molecular shape Brainly. The effect of lone pairs on molecular geometry is evident by looking at methane ammonia and water all with four electron groups. What is the correct name for N₂O₄.
In the molecule they assume a shape a geometry that will minimize electronic. The shapes of these molecules can be predicted from their Lewis structures however with a model developed about 30 years ago known as the valence-shell electron-pair repulsion VSEPR theory. Want this question answered.
How does molecular polarity influence molecules in liquids and solids. The single and the triple bond. Apex Chemistry - 72 26 Terms.
The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an. It can predict the shape of nearly all compounds that have a central atom as. 7 Why is the shape of a molecule important.
Apex Chemistry - 71 69 Terms. The VSEPR theory assumes that each. There is no direct relationship between the formula of a compound and the shape of its molecules.
The shape of a molecule is determined by the repulsion of electron pairs. 9 Why do molecular compounds differ in shape. Valence shell electron pair repulsion theory VESPER for short.
Valence Shell Electron Pair Repulsion is a system that predicts the electronic orbital pair geometry and the molecular geometry shape of a molecule based on the number of bonding and nonbonding electron pairs of a central atom. Valence Shell Electron Pair Repulsion. VSEPR Theory and Predicting the Shape of Molecules Valence shell electron pair repulsion VSEPR theory can be used to predict the shape of molecules.
Valence Shell Electron Pair Repulsion Theory. Valence shell electron pair repulsion theory vesper for short classifies molecules on the basis of the number of electron pairs bonding and lone pairs associated with the central atom. Be notified when an answer is posted.
11 What is the. Advertisement Advertisement correatp0525 correatp0525 Answer. This theory uses the concept of _____ charges in this case the _____ charged electrons repelling each other.
8 What is the other term for the model used to predict the shape of a molecule. The shape of a molecule.

7 6 Molecular Structure And Polarity Chemistry

Comments
Post a Comment